Wearable ultrasound technology from ZetrOZ Systems meets patients' need for for drug-free, non-invasive treatment extending healing to the home
TRUMBULL, Conn., November 11, 2021 (Newswire.com) - What do chiropractors' patients want in their treatment? The better questions are what don't they want in treatments, and what do they want between treatments, according to ZetrOZ Systems' survey of practitioners at one of the largest national conventions of chiropractors.
ZetrOZ, makers of the sam® Sustained Acoustic Medicine device, presented its technology at the Florida Chiropractic Association's national conference in August. Christopher Gardner, Chief Business Officer for ZetrOZ, spoke directly with more than 70 chiropractors to learn what their patients were seeking in their treatment.
"The overwhelming majority said their patients sought drug-free and non-invasive treatment solutions," Gardner said. "But importantly, 100 percent of the chiropractors I spoke with said their patients want to know what they can do between visits to support their recovery."
That's where ZetrOZ and sam® come in. sam® is a long-duration, daily, wearable pain relief ultrasound device that can eliminate the need for pain medication and surgery when prescribed to treat soft tissue injuries. sam® has been validated in more than 30 clinical studies and over 300,000 patients have been treated successfully. The therapy is widely known in the field of orthopedics and sports medicine but less so in the chiropractic community.
Gardner's experience at the convention inspired him to write an informative article for the chiropractic community addressing the most pressing questions from chiropractors about how sam® can help them to offer more to their patients and, ultimately, to elevate their practices.
Gardner's article answers questions such as whether sam® requires a prescription, and what benefits sam® provides beyond those of traditional in-office ultrasound therapy. As an FDA-approved Class II medical device, sam® does require a prescription from a licensed healthcare professional.
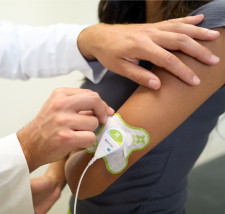
However, the technology has benefits unavailable from in-office ultrasound treatments. Because the FDA has cleared sam® for self-administration, and the device can be worn discreetly, patients can go about daily activities while getting treatment, making treatment readily accessible and easy to regularize.
Additionally, sam® uses long-duration continuous 3MHz ultrasound rather than the short 15-minute 3MHz ultrasound typically provided in clinical settings. Traditional ultrasound produces deep soothing heat, good for therapeutic benefits requiring pain relief, but also requires the ultrasound unit to be continuously applied by the healthcare provider.
In contrast, the ZetrOZ sam® system uses a wearable patch to deliver long-duration ultrasound for up to four hours daily. The home-use and wearable device produces deep sustained delivery of ultrasound to promote soft tissue healing. The patented treatment algorithm and technology let the ultrasound device be placed safely on the body for long periods of time — allowing sam® to be a small, wearable device. Current research also indicates that ultrasound applied for longer periods of time produces better results, providing not only pain relief but also accelerating the body's healing processes.
Studies show that daily, sustained acoustic medicine accelerates soft tissue injury healing, and provides symptomatic relief of knee pain related to osteoarthritis and back pain related to herniated disks. The ZetrOZ technology was proven to provide pain reduction in a randomized, double-blind, placebo-controlled clinical trial, published in 2020 in the Journal of Pain Research. A 2020 study published in the Global Journal of Orthopedics Research found 87 percent of users demonstrated improved function after use of the ZetrOZ samⓇ device.
Gardner notes that surgeons, physicians, physical therapists, sports clinics, and professional and college athletics programs have discovered the benefits of the ZetrOz technology. ZetrOZ clients and patients say the technology gets athletes back in the game sooner and employees back to work more quickly. One study found that worker's compensation claims are reduced by an average of $27,000 when those injured used sam®.
Results like these are why large organizations are recognizing and adopting the sam® technology. Recently, a national network of Department of Veterans Affairs healthcare centers have employed the ZetrOz technology. Gardner's article lays out the benefits specifically for chiropractors and their patients.
"The sam® device is a natural fit for chiropractors' treatment regimens," Gardner said. "The technology is designed for outpatient use, and beneficial for rehabilitation and pain management. The issue is just one of introducing the device to the chiropractic community because they absolutely understand the therapeutic benefits sam® offers in helping heal soft tissue injuries and in pain management."
About ZetrOZ Systems
ZetrOZ Systems is an FDA cGMP and ISO 13585 medical technology company headquartered in the southern coastal region of Connecticut. The organization also has manufacturing facilities across the United States. ZetrOZ Systems produced UltrOZ®, sam®Sport and sam®Pro 2.0 to provide safe and effective treatment options for prevalent conditions such as arthritis. Learn more at https://zetroz-systems.newswire.com.
Media Contact:
Bianca Facey
(203) 577-7588 (Direct)
bianca@newswire.com
www.Newswire.com
Source: ZetrOZ Systems
Share:
