Study shows treatment with low-intensity, continuous ultrasound reduced patients’ pain and need for pain medication.
TRUMBULL, Conn., June 26, 2024 (Newswire.com) - Nearly one out of four Americans (23.3%) suffer from chronic low back pain. A new study finds that low-intensity continuous ultrasound, such as ZetrOZ Systems’ sustained acoustic medicine (sam®) technology, alleviates the symptoms of chronic lower back pain and reduces the need for pain medication.
The study, presented at last month’s 2024 Annual Meeting of the American College of Sports Medicine, examined the effect of low-intensity continuous ultrasound on chronic low back pain and how it can alleviate symptoms of chronic low back pain (CLBP) during a randomized, double-blind, placebo-controlled trial.
The researchers1 found that having daily low-intensity continuous ultrasound (LICUS) treatment significantly eases clinical symptoms of chronic lower back pain compared to placebo treatment. LICUS is considered a safe and non-invasive therapeutic option for patients with CLBP.
“We’re pleased to see another study affirming how our sam® wearable ultrasound unit accelerates tissue healing and relieves pain, especially for people who suffer from low back pain,” George K. Lewis, Ph.D., founder and CEO of ZetrOZ Systems. “Our sam® devices have a proven track record of treating soft tissue injury successfully and helping people feel less pain and be more active.”
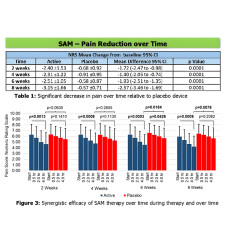
The study included 65 patients (26 men, 39 women, average age 47) who had been suffering from CBLP for over three months. They were assigned to a LICUS or placebo treatment with baseline pain levels and medication usage recorded over two weeks before treatment.
Participants underwent a daily four-hour lower lumbar region treatment with either active or placebo treatment for eight weeks. They maintained daily diaries and reported biweekly pain levels on the numeric rating scale (NRS), global rating of change (GROC), medication reduction, physical activity, and functionality.
The LICUS-treated group demonstrated significant reductions in pain levels (2.30-3.15 NRS scale) at specific intervals over eight weeks over the placebo method. The pain reduction was considerable in the first two weeks and decreased gradually over the next six weeks. After eight weeks, the LICUS-treated group had a reduction of 2.57 points on the NRS scale and a 3.48 GROC score improvement with a much lower difference of 22.5% in daily medication use of morphine (15.2 mg equivalent dosage).
The researchers concluded: “Daily LICUS treatment significantly alleviates clinical symptoms of CLBP relative to placebo treatment. LICUS is a safe and non-invasive therapeutic option for patients with CLBP.”
The new study is the latest body of research documenting the effectiveness of ZetrOZ's sustained acoustic medicine technology in treating these types of conditions, documented in 42 peer-reviewed publications and 20 Level 1-5 clinical studies and thousands of patients treated with sam® every day. The sam® line of devices is cleared by the U.S. Food & Drug Administration for treating conditions such as knee osteoarthritis, patellar and shoulder tendinopathy, and chronic back pain.
To learn more, please visit https://zetroz.com. For more information on ZetrOZ Systems, please visit www.samrecover.com.
1) Jason Krystofiak1, Ralph Ortiz2, Thomas Motyka3, Stephine Petterson4, Kevin Plancher5. 1RWJ Barnabas Health, Livingston, NJ. 2 Cayuga Medical Center, Ithaca, NY. 3Campbell University, Buies Creek, NC. 4Orthopedic Foundation, Stamford, CT. 5 Albert Einstein College of Medicine, Bronx, NY. (Sponsor: Amy Valasek, FACSM)
About ZetrOZ Systems
ZetrOZ Systems is leading healing innovations in sports medicine, developing wearable bioelectronic devices to deliver sustained acoustic medicine (sam®). Researched and funded by the federal government, ZetrOZ is built on the proprietary medical technology of 46 patents and is the exclusive manufacturer and developer of the sam® product line, designed to treat acute and chronic musculoskeletal conditions.
Source: ZetrOZ Systems
Share:
